NIC Excellence Project 2023/1
Structural dynamics of apo, agonist-, and antagonist-bound full-length ETR1

John von Neumann Excellence Project 2023/1
Prof. Holger Gohlke (Universität Düsseldorf)
The small molecule ethylene is a gaseous plant hormone known to induce various developmental processes in plants such as seed germination, senescence, and fruit ripening after binding to the plant receptor ETR1 (ethylene response 1) in its transmembrane sensor domain (TMD). The TMD obtains its high affinity and specificity for the chemically simple ethylene molecule through an essential copper cofactor, which also binds in the TMD. Involved in post-harvest spoilage through fruit ripening, ethylene signaling is an agronomically relevant reaction, and understanding and modulating it is a broad field of research.
Previously, we aimed to model copper chaperone/ETR1 complexes to study the mechanism of copper transfer at the atomistic level. We generated chaperone/ETR1 complexes using protein-protein docking and steered MD simulations, respectively. Building on this, we now also want to address the question of how copper is transported from the chaperone/ETR1 complex to the binding site in the TMD. Since copper was shown to be essential for ethylene binding and receptor function, these studies will provide insights into the role and transport of copper for ethylene receptor biogenesis and ethylene perception in plants.
Ethylene receptors function as negative regulators of the ethylene signaling pathway upon activation. Ethylene diffuses throughout the plant or to surrounding plants and binds to the TMD of ETR1 located in the ER membranes. Receptors bound to ethylene undergo conformational changes and therefore fail to activate downstream targets, which finally triggers the ethylene response of the plant and initiates the ripening of fruits. Many strained alkenes, such as 1-methylcyclopropene (1-MCP) are proven to be effective antagonists of ethylene responses that target ethylene receptors and therefore prevent fruit ripening. So far, no mechanistic model has been put forward for how ethylene binding affects ETR1 receptor structure and/or dynamics.
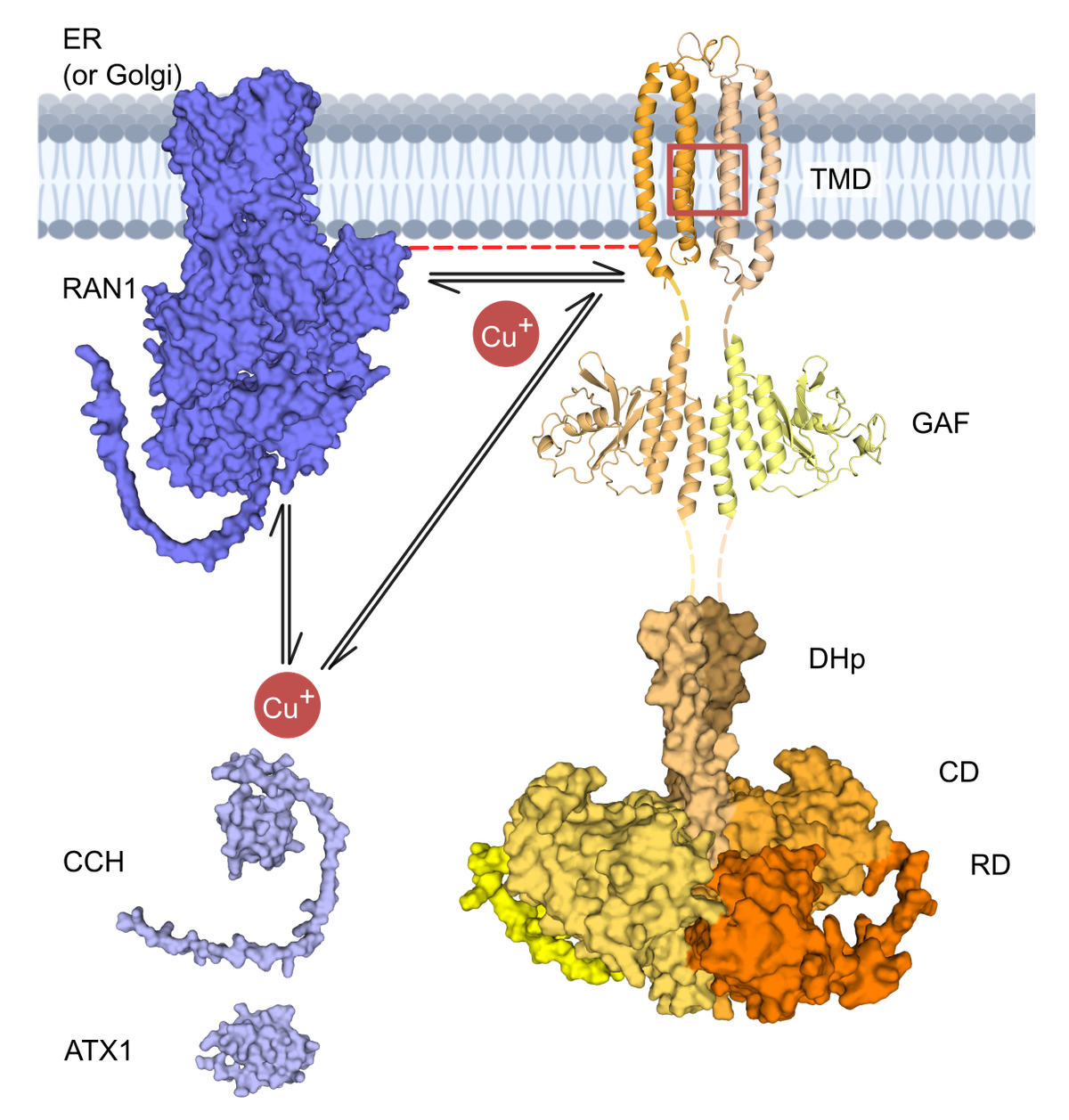
In preliminary work, we predicted and experimentally validated a model of the ETR1 TMD, characterized the ethylene binding site, generated an ETR1:Cu(I):ethylene TMD model, developed parameters of ethylene or 1-MCP binding to Cu(I) employing a bonded model, and performed all-atom MD simulations of the unbound, ethylene-bound, or 1-MCP-bound ETR1_TMD model. We now want to build upon these results and generate a full-length ETR1 structural model, to scrutinize how the ethylene signal is transduced throughout the receptor and investigate the structural dynamics of apo, ethylene-, and 1-MCP-bound ETR1 using molecular simulations in the JUWELS Booster module. The obtained data shall lead to experimentally testable hypotheses as to how ethylene binding causes conformational changes of ethylene receptors and how antagonists, such as 1‑MCP, can block ethylene activity. Our long-term perspective is that these studies will provide the basis for investigating more complex mechanisms of signaling and regulation of ethylene receptors.




