Energy materials and batteries
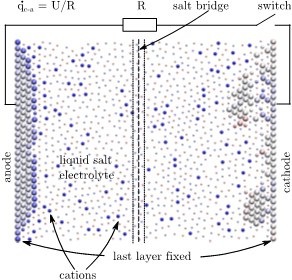
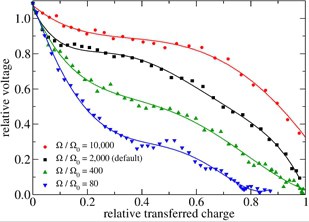
Batteries are critical components in addressing today’s energy challenges. Yet atomic-scale simulations of batteries during discharge or recharge have remained elusive. What happens precisely at the electrode surfaces when ions leave the anode or reach the cathode? To get insight into such questions we applied our redox-reactions potentials to a nano-scale model of a battery. In these simulations, we can correlate fluctuations of individual discharge characteristics with the atomic-scale processes.
So far, these simulations were based on simple, “generic” potentials, which are not element specific. To better address material-dependent peculiarities, we started to improve conventional force fields, in particular in the context of quasi-atom-theory based potentials. We consider SMEAM [J. Jalkanen and M. H. Müser, Model. Simul. Mater. Sc. Eng. 23, 074001 (2015)] as a promising route towards this goal.
References:
W. Dapp and M. H. Müser, Redox reactions with empirical potentials: Atomistic battery discharge simulations,
J. Chem. Phys. 139, 064106 (2013),
DOI: 10.1063/1.4817772
J. Jalkanen and M. H. Müser, Systematic analysis and modification of embedded-atom potentials: case study of copper,
Model. Simul. Mater. Sc. Eng. 23, 074001 (2015),
DOI: 10.1088/0965-0393/23/7/074001





